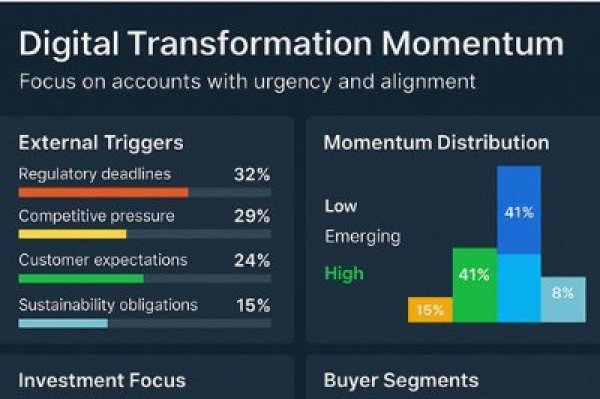
Results for 'pharmaceutical regulation'

New Book Unites Oncology’s Brightest Minds To Innovate Cancer Cures
Sep 8th • 5 mins read

“Extreme Interviewing” – MSL Interview Tips and Insights from Medical Affairs Leaders
Jul 8th • 1 min read






Accelerated drug approvals in oncology: Pros and cons
Sep 13th • 4 mins read

Past, Current, and Future Cancer Clinical Research Collaborations: The Case of the European Organisation for Research and Treatment of Cancer
Aug 15th • 8 mins read

Biosimilars in oncology: key role of nurses in patient education
Jun 14th • 10 mins read

Clinical development success rates and social value of pediatric Phase 1 trials in oncology
Jun 20th • 28 mins read

Real-World Evidence: Bridging Gaps in Evidence to Guide Payer Decisions
Jun 17th • 6 mins read

The regulatory landscape of precision oncology laboratory medicine in the United States - Perspective on the past 5 years and considerations for future regulation
May 21st • 8 mins read

Sponsorship of oncology clinical trials in the United States according to age of eligibility
Apr 28th • 8 mins read
EHA evaluation of the ESMO-Magnitude of Clinical Benefit Scale version 1.1 (ESMO-MCBS v1.1) for hematological malignancies
Jan 19th • 20 mins read

Prediction of Drug Approval After Phase I Clinical Trials in Oncology: RESOLVED2
Sep 19th • 12 mins read

Association of National Cancer Institute-Sponsored Clinical Trial Network Group Studies With Guideline Care and New Drug Indications
Sep 3rd • 17 mins read
